
Our Future
BRINGING BACK THE LIGHT OF HOPE
Our Future
BRINGING BACK THE LIGHT OF HOPE
We work every day to bring back the light of hope to millions of families worldwide who are struggling with lives disrupted by the most complex retinal diseases.
OUR ACTIVE PIPELINE
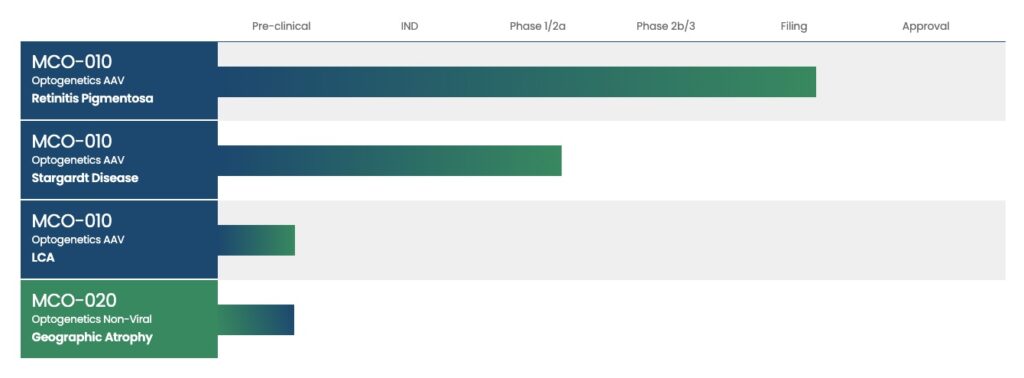
Pre-clinical
IND
Phase 1/2a
Phase 2b/3
Filing
Approval
MCO-010
Optogenetics AAV*
Retinitis Pigmentosa
MCO-010
Optogenetics AAV
Stargardt Disease
MCO-010
Optogenetics AAV
LCA
MCO-020
Optogenetics Non-Viral
Geographic Atrophy
*adeno-associated virus
FDA has granted MCO-010 orphan and fast track designations, with the potential to be granted priority review for Retinitis Pigmentosa and Stargardt disease.1
- If eligibility criteria are met for designation, FDA will grant priority review designation at the time of filing of a marketing application.
CURRENT DISEASE TARGETS
Our clinical programs for Retinitis Pigmentosa and Stargardt disease are focused to show vision improvement for patients that have no current treatment options.
Retinitis Pigmentosa (RP)

Retinitis Pigmentosa is one of the leading causes of blindness in the US working age population.
RP is caused by mutations that have been found in over 100 different genes, each of which contribute to the development and functioning of the retina.
RP is characterized by the progressive degeneration and dysfunction of the retina’s rod and cone photoreceptor cells, which are responsible for converting light into electrical signals that the brain interprets as vision.
Initially, RP affects peripheral vision leading to tunnel vision and in later progression impacts central vision. RP typically manifests during childhood or adolescence, but it can also present in adulthood.
RP leads to progressive, irreversible vision loss with over 100,000 total RP patients and more than 25,000 legally blind RP patients in the US and growing.
Nanoscope Clinical Trials for RP
MCO-010 for RP
REMAIN Study
STATUS: Active, not recruiting
Non-interventional long term follow-up study of participants previously enrolled in the RESTORE Phase 2b trial of MCO-010 for advanced RP.
Stargardt Disease (SD)
Stargardt disease, also known as Stargardt macular dystrophy or juvenile macular degeneration, is typically diagnosed during childhood or adolescence, although it may be identified in adulthood.
SD is an inherited eye disorder that affects the macula in the center of the retina. The macula is responsible for sharp central vision.
SD symptoms include central vision loss, blurry or distorted vision, difficulty recognizing faces, impaired color vision, and a blind spot in the central field of vision. The peripheral vision, however, usually remains intact.
Patients with advanced SD unfortunately arrive at the same state of profound vision loss due to photoreceptor degradation as patients with RP.
Nanoscope Clinical Trials for SD
MCO-010 for SD
SUSTAIN Study
STATUS: Active, not recruiting
Non-Interventional Long Term Follow-up Study of Participants Previously Enrolled in the STARLIGHT Ph2 Trial of MCO-010 for Stargardt disease.
