Nanoscope Awarded $1.5M Phase2B SBIR Grant to Advance Ambient Light Activatable Opsin Gene Therapy to Restore Vision for AMD Patients
BEDFORD, TX (September 14, 2021)—Nanoscope researchers were awarded a $1.5 million Phase2b Small Business Research Innovation (SBIR) grant by the National Eye Institute (NEI)of the National Institutes of Health (NIH) to advance the company’s ambient light activatable optogenetic gene therapy for age-related macular degeneration(AMD).
“Winning the NIH grant via a highly competitive review process is a testament to the innovativeness and clinical significance of our first-in-class optogenetic therapy based on our Multi-Characteristic Opsin (MCO),which is a patent-protected ambient light activatable protein for restoration of vision in people with AMD,”said Sulagna Bhattacharya, CEO of Nanoscope.“Our goal is to apply our therapy to relieve the suffering of millions of people worldwide with AMD.”’
Nanoscope’s lead MCO gene therapy, MCO-010, is in a late-stage Phase 2b trial for retinitis pigmentosa (RP) in the US. MCO-010 has orphan drug designations for RP and Stargardt disease from the US Food and Drug Administration. A Phase 1/2a trial of MCO-010 showed patients blinded by RP experienced clinically meaningful vision restoration.
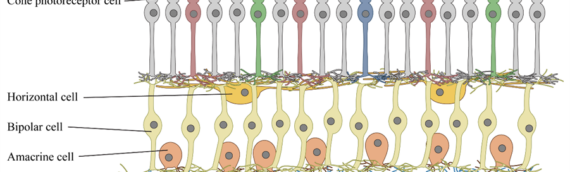
Nanoscope’s optogenetic gene therapy uses a proprietary AAV2 vector to deliver MCO genes into bipolar retinal cells where they express MCOs engineered to be fast and polychromatic, enabling vision in different color environments. The MCOs reprogram bipolar cells to act like photoreceptor cells damaged not only by AMD, but also the full range of inherited retinal disorders (IRDs) regardless of underlying disease-causing gene mutations. The therapy involves a single intravitreal injection and can be administered in a medical office setting.
“Our preliminary data show MCO therapy can be applied in a mutation-independent manner for specific IRDs and can serve as a retinal disease-agnostic platform therapy,” said Samarendra Mohanty, PhD, Principal Investigator of the recently awarded NIHgrant and Nanoscope’s President and Chief Scientific Officer.“ With this grant we can accelerate advancement of our MCO therapy for geographic atrophies of the macula for juveniles as well as adults.”
About Nanoscope Therapeutics Inc.
Nanoscope Therapeutics is developing optogenetic therapies for giving sight to the millions of blind individuals suffering from retinal degenerative diseases, for which no cure exists. The company’s pipeline includes optogenetics based retinal regeneration therapy for patients with RP, Stargardt disease, and other IRDs as well as age-related macular degeneration.
Contact:
Charles Craig
Opus Biotech Communications
pr@nanostherapeutics.com
404-245-0591