Nanoscope Therapeutics is advancing gene therapy using ambient light-sensitive molecules, bringing hope for renewed sight to millions of people blinded by retinal degenerative diseases for which no cure exists.
Hear what a Stargardt Macular Degeneration patient in our STARLIGHT Clinical Trial has to say about her experience.
Roy, a patient in our RESTORE Clinical Trial for Retinitis Pigmentosa began to experience vision loss 20 years ago. After MCO-010 treatment Roy saw colors he had not seen in years and is now determined to participate in all aspects of his community.
Richard, a patient in our RESTORE Clinical Trial for Retinitis Pigmentosa, talks about the changes MCO-010 treatment has made in his life.
Hear how John, a patient in our RESTORE Clinical Trial for Retinitis Pigmentosa feels about his experience two years after being enrolled in the study.
Jeff, a patient in our STARLIGHT Clinical Trial for Stargardt Macular Degeneration began to experience vision loss as a young boy. Today, Jeff is seeing more of the world and feeling positive about the future.
Latest News from Nanoscope
Press Release: October 10, 2024
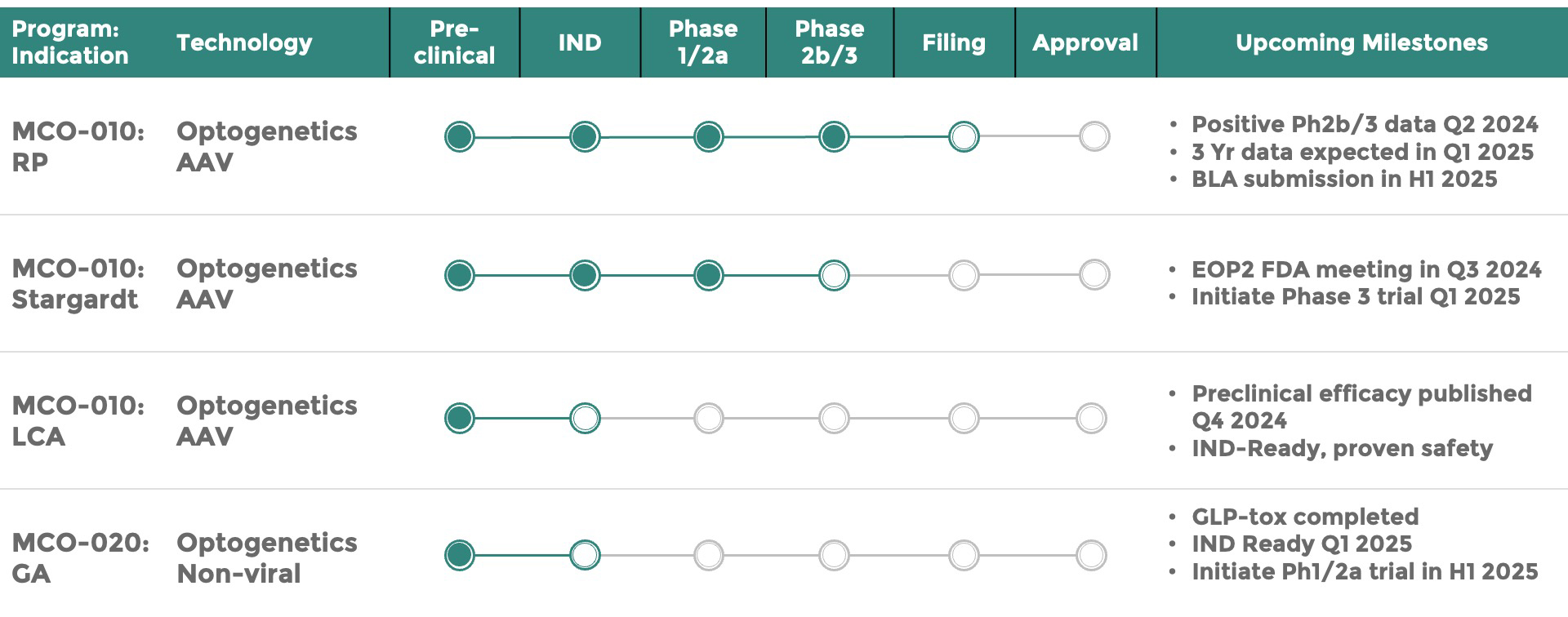
Nanoscope Announces Plans to Submit BLA for MCO-010 to Treat Retinitis Pigmentosa
Press Release: September 12, 2024
Nanoscope Therapeutics Announces End-of-Phase 2 Meeting with U.S. FDA and Plan to Initiate a Phase 3 Clinical Trial of MCO-010 to Treat Stargardt Macular Degeneration
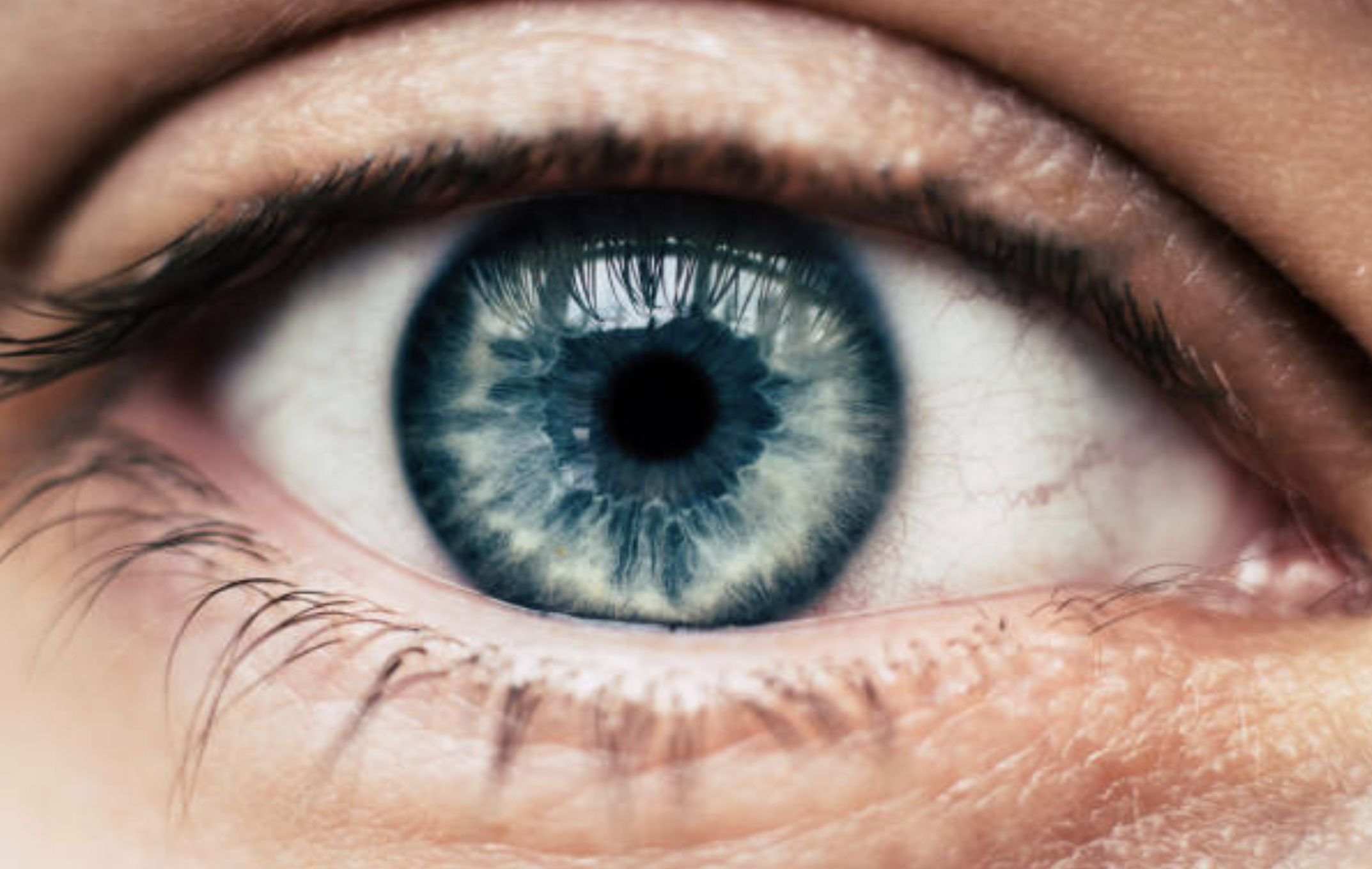
Nanoscope is focused on proprietary ambient light-activated optogenetic therapy to restore vision in people suffering from various inherited retinal degenerative diseases including Retinitis Pigmentosa, Stargardt, and dry AMD.
Genetic retinal degenerative diseases cause vision loss as cells of the retina gradually become dysfunctional. Nanoscope’s proprietary gene therapies correct the condition by delivering light-sensitive molecules, called Multi-Characteristic Opsins (MCO), into retinal cells.
LEARN ABOUT OUR TECHNOLOGY
Our lead gene therapy product in clinical trials for retinitis pigmentosa will be the first therapy to correct the condition and is applicable for patients with complete or partial degeneration of the retina.
Pioneering a New Wave of Optogenetic Therapeutics for Vision Restoration
Nanoscope News & Events
-
 Pharmaceutical Industry Veteran, Ashish Patel, PhD, Joins Nanoscope as Senior Vice President of Sales and MarketingMarch 27, 2025
Pharmaceutical Industry Veteran, Ashish Patel, PhD, Joins Nanoscope as Senior Vice President of Sales and MarketingMarch 27, 2025 -
 Nanoscope Announces Publication of Clinical Data on Vision Restoration in Retinitis PigmentosaMarch 24, 2025
Nanoscope Announces Publication of Clinical Data on Vision Restoration in Retinitis PigmentosaMarch 24, 2025 -
 Nanoscope Therapeutics Announces the Appointment of Ademola Daramola, D.Sc., as Vice President, Quality & ComplianceMarch 20, 2025
Nanoscope Therapeutics Announces the Appointment of Ademola Daramola, D.Sc., as Vice President, Quality & ComplianceMarch 20, 2025 -
 Nanoscope Therapeutics to Present at the H.C. Wainwright 3rd Annual BioConnect ShowcaseMarch 5, 2025
Nanoscope Therapeutics to Present at the H.C. Wainwright 3rd Annual BioConnect ShowcaseMarch 5, 2025 -
Verana Health and Nanoscope Therapeutics Announce Collaboration to Accelerate Retinitis Pigmentosa ResearchFebruary 25, 2025
-
 Nanoscope Therapeutics to Present at the BIO CEO and Investor ConferenceFebruary 6, 2025
Nanoscope Therapeutics to Present at the BIO CEO and Investor ConferenceFebruary 6, 2025 -
 Nanoscope Therapeutics Announces Presentations at Upcoming Medical MeetingsFebruary 4, 2025
Nanoscope Therapeutics Announces Presentations at Upcoming Medical MeetingsFebruary 4, 2025 -
 Nanoscope Therapeutics to Present at the 21st Annual Advanced Therapies Week ConferenceJanuary 20, 2025
Nanoscope Therapeutics to Present at the 21st Annual Advanced Therapies Week ConferenceJanuary 20, 2025


